What Is The Change In The Specific Entropy Of Superheated R134a At 130
Contents
Superheated Steam
An explanation of the properties and uses of superheated steam (such equally for electricity generation). Including explanations of the Rankine and Carnot thermodynamic cycles, superheated steam tables and the Mollier (H-S) chart.
If the saturated steam produced in a boiler is exposed to a surface with a college temperature, its temperature will increase above the evaporating temperature.
The steam is then described as superheated by the number of temperature degrees through which information technology has been heated above saturation temperature.
Superheat cannot be imparted to the steam whilst it is still in the presence of water, equally any additional heat simply evaporates more than water. The saturated steam must be passed through an boosted heat exchanger. This may be a second oestrus exchange stage in the boiler, or a carve up superheater unit. The primary heating medium may be either the hot flue gas from the banality, or may be separately fired.
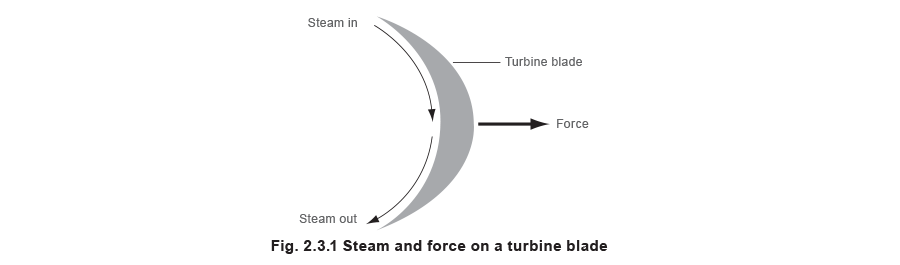
Superheated steam has its applications in, for example, turbines where the steam is directed past nozzles onto a rotor. This causes the rotor to turn. The energy to make this happen can only have come from the steam, and so logically the steam has less energy after information technology has gone through the turbine rotor. If the steam was at saturation temperature, this loss of energy would cause some of the steam to condense.
Turbines accept a number of stages; the frazzle steam from the first rotor will be directed to a 2d rotor on the aforementioned shaft. This means that saturated steam would get wetter and wetter equally it went through the successive stages. Not but would this promote waterhammer, only the water particles would crusade severe erosion within the turbine. The solution is to supply the turbine with superheated steam at the inlet, and use the energy in the superheated portion to drive the rotor until the temperature/pressure level conditions are close to saturation; and and then exhaust the steam.
Some other very of import reason for using superheated steam in turbines is to amend thermal efficiency.
The thermodynamic efficiency of a rut engine such every bit a turbine, may exist adamant using one of two theories:
(Note: The values used for the temperature and energy content in the post-obit examples are from steam tables)
Ii Theories
Example 2.three.1
A turbine is supplied with superheated steam at 90 bar a @ 450 °C.
The exhaust is at 0.06 bar a (partial vacuum) and 10% wet.
Saturated temperature = 36.2 °C.
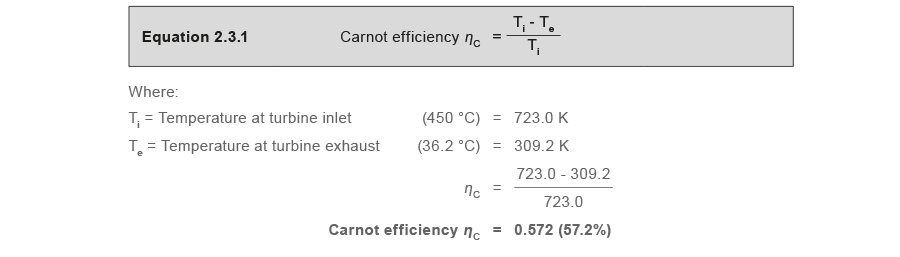
ii.three.1.1 Determine the Carnot efficiency (ηC)
2.three.ane.2 Decide the Rankine efficiency (ηR)
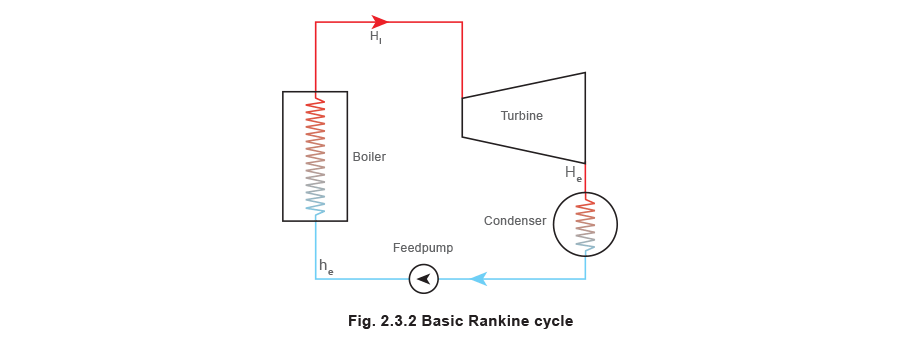
For the theoretical Rankine Cycle, Figure ii.3.2, it is assumed that there are no frictional losses in the turbine, perfect expansion of the steam occurs in the turbine (isentropic), and ignores energy added by the feedpump returning condensate to the boiler.
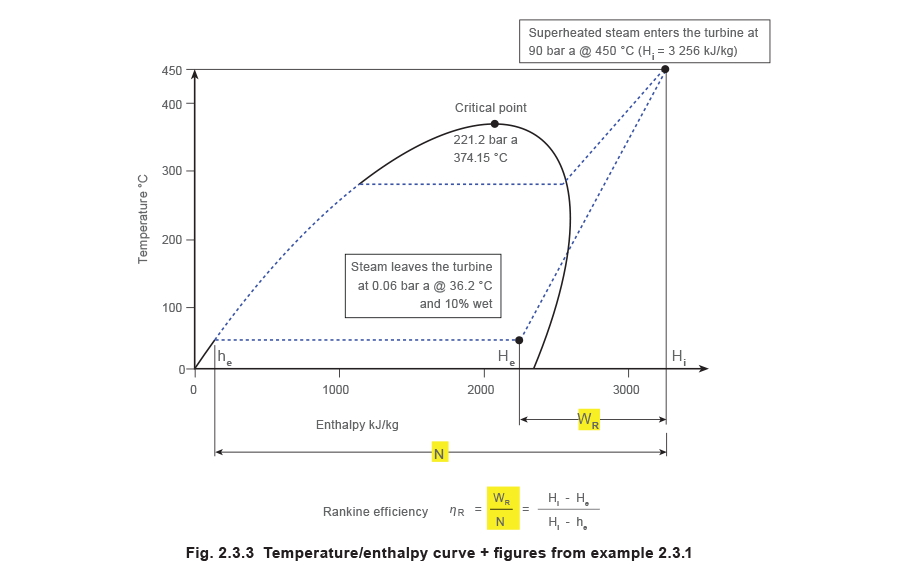
Using Example 2.three.1 where:
- A turbine is supplied with superheated steam at 90 bar a @ 450 °C.
- The exhaust is at 0.06 bar a (partial vacuum) and 10% wet.
- Saturated temperature = 36.2 °C.
This data can be plotted on the temperature/enthalpy curve equally illustrated in Figure two.3.3:

Examination of thursdaye figures for either of the cycles indicates that to achieve loftier efficiency:
- The temperature or free energy at the turbine inlet should exist as high as possible. This means as high a force per unit area and temperature as is practically possible. Superheated steam is the simplest fashion of providing this.
- The temperature or energy in the exhaust must be equally low as possible. This ways as low a pressure and temperature every bit is practically possible, and is commonly achieved by a condenser on the turbine frazzle.
Notes:
- The figures calculated in Examples 2.3.ane.1 and 2.three.1.2 are for thermodynamic efficiency, and must not exist dislocated with mechanical efficiency.
- Although the efficiency figures announced to be very depression, they must not be viewed in isolation, simply rather used to compare ane type of heat engine with another. For case, gas turbines, steam engines and diesel engines.
Superheated steam tables
The superheated steam tables brandish the backdrop of steam at various pressures in much the same way as the saturated steam tables. However, with superheated steam at that place is no direct relationship between temperature and force per unit area. Therefore at a particular pressure level it may be possible for superheated steam to exist at a broad range of temperatures.
In general, saturated steam tables give gauge pressure, superheated steam tables give absolute pressure level.
| Absolute pressure bar a | Units | Temperature (°C) | |||||
| one.013 | 150 | 200 | 250 | 300 | 400 | 500 | |
| vg (m3/kg) | i.912 | 2.145 | 2.375 | two.604 | 3.062 | 3.519 | |
| ug (kJ/kg) | two 583 | 2 659 | 2 734 | 2 811 | 2 968 | 3 131 | |
| hg (kJ/kg) | 2 777 | 2 876 | 2 975 | 3 075 | 3 278 | 3 488 | |
| southwardyard (kJ/kg Thou) | 7.608 | seven.828 | 8.027 | 8.209 | viii.537 | 8.828 | |
Example 2.iii.ii
How much more heat does superheated steam with a temperature of 400 °C and a pressure of 1.013 bar a (0 bar g) have than saturated steam at the same pressure?
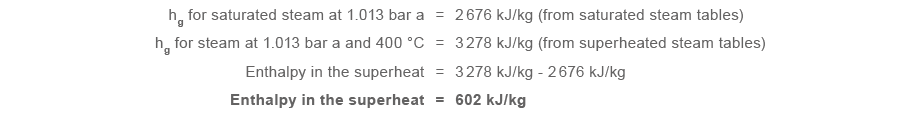
This may sound a useful increment in free energy, simply in fact it will really make life more difficult for the engineer who wants to employ steam for heating purposes.
From the energy in the superheat shown, the specific estrus capacity can be determined by dividing this value past the temperature difference between saturation temperature (100 °C) and the superheated steam temperature (400 °C):
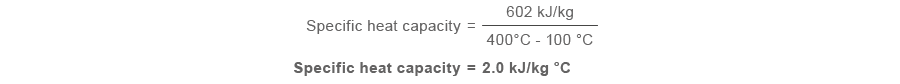
Nonetheless, unlike the specific oestrus chapters of h2o, the specific heat capacity for superheated steam varies considerably with pressure and temperature and cannot be taken as a constant.
The value of 2.0 kJ/kg °C given above is therefore only the mean specific rut capacity over the specified temperature range for that pressure level.
There is no directly relationship between temperature, pressure and the specific rut capacity of superheated steam. There is, even so, a general tendency towards an increase in specific oestrus chapters with increasing pressure at low degrees of superheat, simply this is non always the case.

Can superheated steam be used in process heat exchangers and other heating processes?
Although not the platonic medium for transferring heat, superheated steam is sometimes used for process heating in many steam plants around the globe, especially in the HPIs (Hydrocarbon Processing Industries) which produce oils and petrochemicals. This is more likely to be considering superheated steam is already available on site for power generation, being the preferred energy source for turbines, rather than because it has any advantage over saturated steam for heating purposes. To be clear on this point, in most cases, saturated steam should be used for oestrus transfer processes, even if it means desuperheating the steam to do so. HPIs oft desuperheat steam to within almost ten degrees of superheat. This small degree of superheat is removed readily in the first part of the heating surface. Greater amounts of superheat are more difficult, and often uneconomic to deal with and (for heating purposes) are all-time avoided.
There are quite a few reasons why superheated steam is not as suitable for procedure heating as saturated steam:
Superheated steam has to cool to saturation temperature before it can condense to release its latent heat (enthalpy of evaporation). The amount of heat given upwards by the superheated steam equally it cools to saturation temperature is relatively small-scale in comparison to its enthalpy of evaporation.
If the steam has only a few degrees of superheat, this small amount of heat is quickly given upwardly earlier information technology condenses. However, if the steam has a large degree of superheat, information technology may take a relatively long time to cool, during which fourth dimension the steam is releasing very lilliputian free energy.
Unlike saturated steam, the temperature of superheated steam is not compatible. Superheated steam has to cool to give up heat, whilst saturated steam changes phase. This means that temperature gradients over the heat transfer surface may occur with superheated steam.
In a heat exchanger, employ of superheated steam tin atomic number 82 to the formation of a dry wall boiling zone, shut to the tube sheet. This dry wall area can rapidly become scaled or fouled, and the resulting high temperature of the tube wall may cause tube failure.
This clearly shows that in estrus transfer applications, steam with a large degree of superheat is of little use considering it:
- Gives up little estrus until it has cooled to saturation temperature.
- Creates temperature gradients over the heat transfer surface as it cools to saturation temperature.
- Provides lower rates of heat transfer whilst the steam is superheated.
- Requires larger heat transfer areas.
So, superheated steam is not equally effective equally saturated steam for rut transfer applications. This may seem strange, considering that the charge per unit of heat transfer across a heating surface is directly proportional to the temperature difference across it. If superheated steam has a college temperature than saturated steam at the same pressure, surely superheated steam should exist able to impart more heat? The reply to this is 'no'. This will now be looked at in more detail.
It is true that the temperature divergence volition have an effect on the rate of heat transfer beyond the rut transfer surface, as clearly shown Equation 2.5.three.
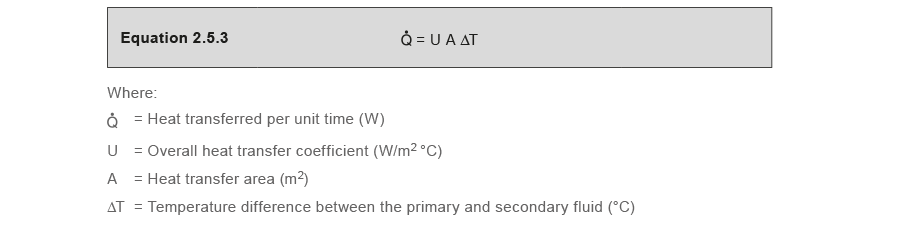
Equation 2.5.3 also shows that heat transfer volition depend on the overall rut transfer coefficient 'U', and the heat transfer area 'A'.
For any single application, the heat transfer area might exist fixed. All the same, the same cannot be said of the 'U' value; and this is the major difference between saturated and superheated steam.
The overall 'U' value for superheated steam will vary throughout the process, just volition ever be much lower than that for saturated steam. It is hard to predict 'U' values for superheated steam, as these volition depend upon many factors, but generally, the higher the degree of superheat, the lower the 'U' value.
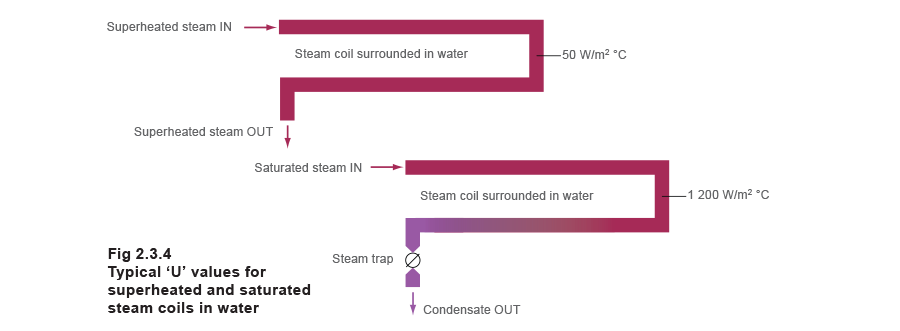
Typically, for a horizontal steam scroll surrounded with water, 'U' values might exist as low as 50 to 100 W/m² °C for superheated steam only 1 200 Westward/grand² °C for saturated steam, as depicted in Figure 2.3.four.
For steam to oil applications, the 'U' values might be considerably less, mayhap as low as 20 Due west/m² °C for superheated steam and 150 West/m² °C for saturated steam.
In a shell and tube heat exchanger, 100 W/thou² °C for superheated steam and 500 W/m² °C for saturated steam tin can be expected. These figures are typical; bodily figures will vary due to other design and operational considerations.
Although the temperature of superheated steam is always higher than saturated steam at the aforementioned pressure, its ability to transfer heat is therefore much lower. The overall effect is that superheated steam is much less constructive at transferring heat than saturated steam at the same pressure. The adjacent Section 'Fouling' gives more detail.
Not merely is superheated steam less constructive at transferring estrus, information technology is very difficult to quantify using Equation 2.v.three, Q̇ = U A ΔT, every bit the temperature of the steam volition fall equally it gives upwards its heat while passing along the heating surface.
Predicting the size of heat transfer surfaces utilising superheated steam is difficult and complex. In practice, the basic data needed to perform such calculations is either not known or empirically obtained, putting their reliability and accuracy in doubtfulness.
Conspicuously, as superheated steam is less constructive at transferring estrus than saturated steam, so any heating surface area using superheated steam would have to be larger than a saturated steam coil operating at the same pressure to evangelize the same heat flowrate.
If there is no choice simply to employ superheated steam, information technology is not possible to maintain steam in its superheated country throughout the heating coil or oestrus exchanger, since as it gives up some of its oestrus content to the secondary fluid, it cools towards saturation temperature. The amount of heat above saturation is quite small compared with the big corporeality available as condensation occurs.
The steam should reach saturation relatively soon in the process; this allows the steam to condense to produce higher heat transfer rates and result in a higher overall 'U' value for the whole coil, come across Effigy 2.3.five.
To assistance to enable this, superheated steam used for heat transfer purposes should not hold more virtually 10 °C of superheat.
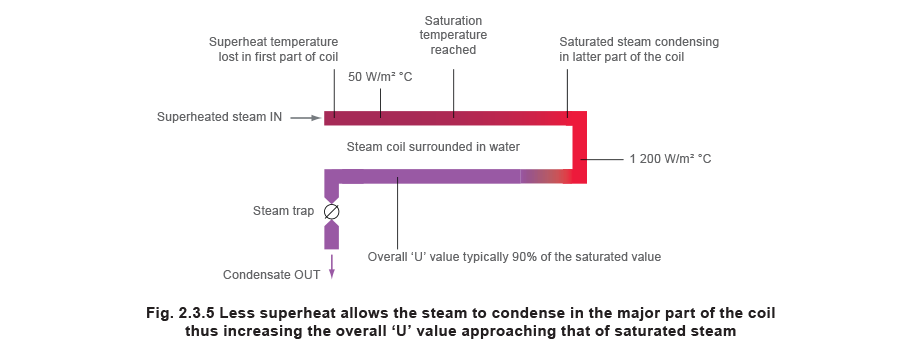
If this is so, it is relatively easy and applied to blueprint a estrus exchanger or a scroll with a heating surface area based upon saturated steam at the aforementioned pressure, past adding on a certain amount of surface area to allow for the superheat. Using this guideline, the start office of a coil volition exist used purely to reduce the temperature of superheated steam to its saturation point. The rest of the whorl will and then exist able to take reward of the higher oestrus transfer ability of the saturated steam. The issue is that the overall 'U' value may not exist much less than if saturated steam were supplied to the roll.
From practical experience, if the extra heating area needed for superheated steam is 1% per 2 °C of superheat, the gyre (or heat exchanger) will be large enough. This seems to work upward to ten °C of superheat. It is not recommended that superheated steam above ten °C of superheat exist used for heating purposes due to the probable disproportionate and uneconomic size of the heating surface, the propensity for fouling past dirt, and the possibility of production spoilage past the high and uneven superheat temperatures.
Fouling
Fouling is acquired by deposits edifice up on the rut transfer surface adding a resistance to heat period. Many process liquids can deposit sludge or scale on heating surfaces, and volition do so at a faster rate at higher temperatures. Further, superheated steam is a dry out gas. Heat flowing from the steam to the metallic wall must pass through the static films adhering to the wall, which resist estrus menstruum.
By contrast, the condensation of saturated steam causes the motility of steam towards the wall, and the release of big quantities of latent heat right at the condensing surface. The combination of these factors means that the overall heat transfer rates are much lower where superheated steam is present, even though the temperature difference betwixt the steam and the secondary fluid is college.
Example 2.3.3 Sizing a tube bundle for superheated steam
Superheated steam at three bar 1000 with ten °C of superheat (154 °C) is to exist used as the master heat source for a crush and tube process oestrus exchanger with a heating load of 250 kW, heating an oil based fluid from 80 °C to 120 °C (making the arithmetic hateful secondary temperature (ΔTAM) 100 °C). Judge the area of principal steam scroll required.
(Arithmetics mean temperature differences are used to keep this calculation simple; in do, logarithmic mean temperatures would be used for greater accuracy. Please refer to Module two.five 'Estrus Transfer' for details on arithmetic and logarithmic mean temperature differences).
First, consider the ringlet if it were heated by saturated steam at 3 bar grand (144 °C).
The 'U' value for saturated steam heating oil via a new carbon steel scroll is taken to be 500 W/m2 °C.
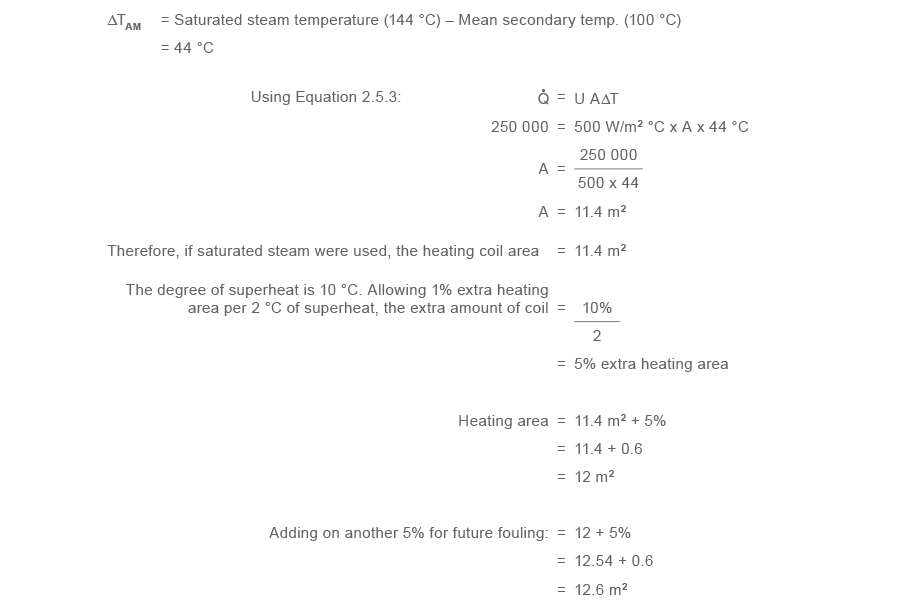
Other applications using superheated steam
All the above applies when steam is flowing through a relatively narrow passage, such equally the tubes in a shell and tube heat exchanger or the plates in a plate heat exchanger.
In some applications, mayhap a drying cylinder in a newspaper auto, superheated steam is admitted to a greater book, when its velocity plummets to very small values. Here, the steam near the wall of the cylinder quickly drops in temperature to well-nigh saturation and condensation begins. The heat menses through the wall is and so the same equally if the cylinder were supplied with saturated steam. Superheat is present only within the 'core' in the steam space and has no discernible effect on heat transfer rates.
At that place are instances where the presence of superheat tin really reduce the performance of a process, where steam is being used every bit a process fabric.
One such process might involve moisture existence imparted to the product from the steam equally information technology condenses, such as, the conditioning of animal feedstuff (meal) prior to pelletising. Here the moisture provided by the steam is an essential part of the process; superheated steam would over-dry the repast and make pelletising difficult.
The effects of reducing steam pressure
In add-on to the use of an additional heat exchanger (generally called a 'superheater'), superheat can also be imparted to steam by allowing information technology to expand to a lower pressure every bit it passes through the orifice of a pressure reducing valve. This is termed a throttling process with the lower force per unit area steam having the same enthalpy (apart from a pocket-sized corporeality lost to friction in passing through the valve) equally the upstream high pressure steam. However, the temperature of the throttled steam will ever exist lower than that of the supply steam.
The land of the throttled steam will depend upon:
- The pressure of the supply steam.
- The land of the supply steam.
- The pressure drop across the valve orifice.
For supply steam below 30 bar grand in the dry saturated country, any drib in pressure will produce superheated steam later on throttling. The caste of superheat volition depend on the amount of pressure reduction.
For supply steam higher up xxx bar g in the dry saturated country, the throttled steam might be superheated, dry saturated, or even wet, depending on the corporeality of pressure drop. For example, dry saturated steam at threescore bar m would have to be reduced to approximately 10.five bar m to produce dry saturated steam. Whatever less of a force per unit area drop will produce wet steam, while whatsoever greater pressure level driblet would produce superheated steam.
Equally, the state of the supply steam at any pressure will influence the state of the throttled steam. For example, moisture steam at a pressure of 10 bar g and 0.95 dryness fraction would need to exist reduced to 0.135 bar one thousand to produce dry saturated steam. Any less of a pressure drop would produce wet steam while any greater pressure drop would superheat the throttled steam.
Case 2.iii.4 Increasing the dryness of wet steam with a control valve
Steam with a dryness fraction (χ) of 0.95 is reduced from vi bar g to 1 bar one thousand, using a pressure reducing valve.
Make up one's mind the steam conditions later the pressure reducing valve.
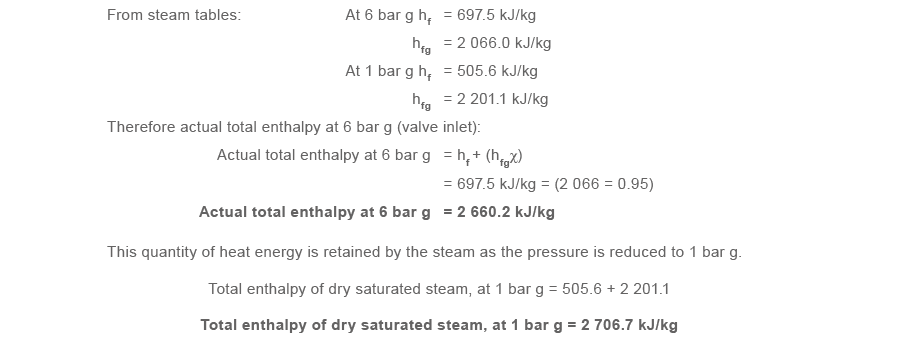
Every bit the actual enthalpy of the steam at 1 bar g is less than the enthalpy of dry saturated steam at 1 bar grand, then the steam is not superheated and even so retains a proportion of moisture in its content.
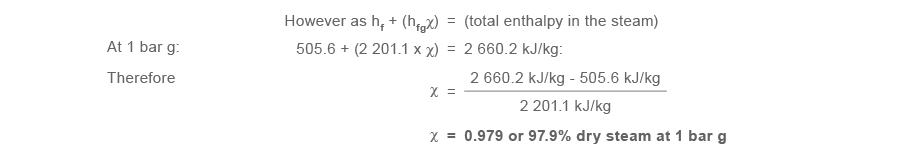
Since the total enthalpy after the pressure reducing valve is less than the total enthalpy of steam at ane bar g, the steam is still moisture.
Example ii.three.5 Superheat created by a command valve
Steam with a dryness fraction of 0.98 is reduced from 10 bar chiliad down to 1 bar k using a pressure reducing valve (as shown in Figure 2.3.vi).
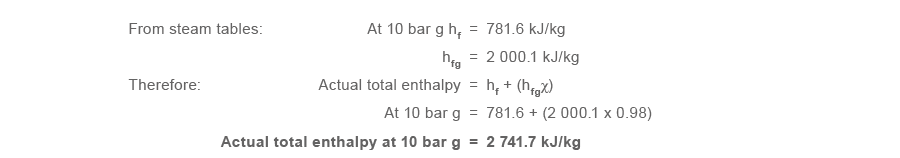
Determine the caste of superheat later the valve.
As in the previous example (2.three.four), the specific enthalpy of dry saturated steam (hg) at 1 bar m is 2 706.7 kJ/kg.
The actual full enthalpy of the steam is greater than the full enthalpy (hg) of dry out saturated steam at 1 bar yard. The steam is therefore non only 100% dry, but also has some degree of superheat.
The backlog free energy = 2 741.vii - 2 706.seven = 35 kJ/kg, and this is used to raise the temperature of the steam from the saturation temperature of 120 °C to 136 °C.
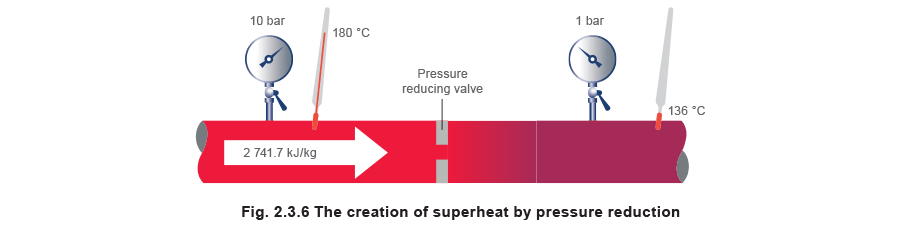
The caste of superheat can be determined either by using superheated steam tables, or by using a Mollier nautical chart.
The Mollier chart
The Mollier chart is a plot of the specific enthalpy of steam confronting its specific entropy (s1000).
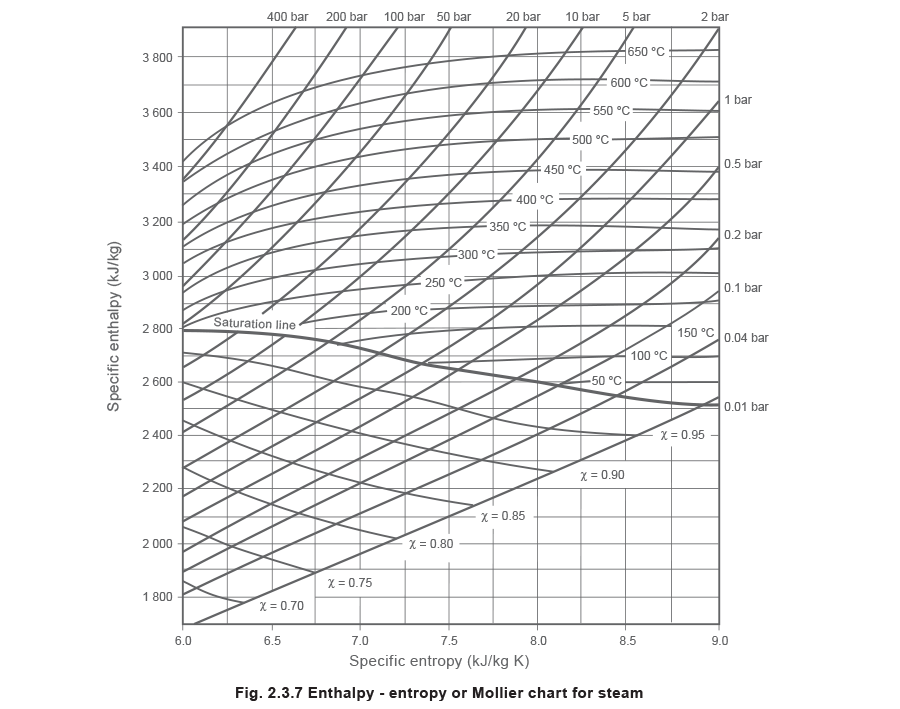
Figure two.3.7 shows a simplified, minor scale version of the Mollier chart. The Mollier chart displays many different relationships between enthalpy, entropy, temperature, force per unit area and dryness fraction. Information technology may appear to be quite complicated, due to the number of lines:
Abiding enthalpy lines (horizontal).
Constant entropy lines (vertical).
The steam saturation curve across the centre of the chart divides it into a superheated steam region, and a wet steam region. At any point above the saturation bend the steam is superheated, and at any point below the saturation curve the steam is wet. The saturation curve itself represents the condition of dry saturated steam at various pressures.
Abiding pressure lines in both regions.
Abiding temperature lines in the superheat region.
Constant dryness fraction (χ) lines in the wet region.
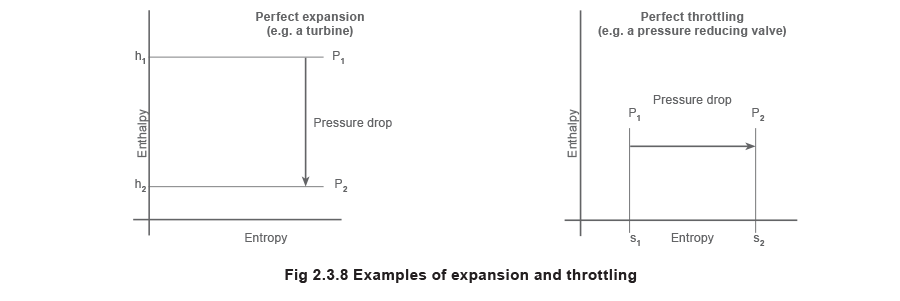
Example 2.3.6 Perfect isentropic expansion resulting in work
Consider the perfect expansion of steam through a turbine. Initially the pressure is 50 bar a, the temperature is 300 °C, and the final pressure is 0.04 bar a.
As the process is a perfect expansion, the entropy remains abiding. The terminal status can then be found by dropping vertically downwards from the initial condition to the 0.04 bar a constant pressure line (meet Effigy 2.iii.9).
At the initial condition, the entropy is approximately 6.25 kJ/kg °C. If this line is followed vertically down until 0.04 bar a is reached, the last status of the steam can be evaluated. At this point the specific enthalpy is 1 890 kJ/kg, and the dryness fraction is 0.72 (see Figure two.three.9).
The final status can also exist determined by using the superheated steam tables.
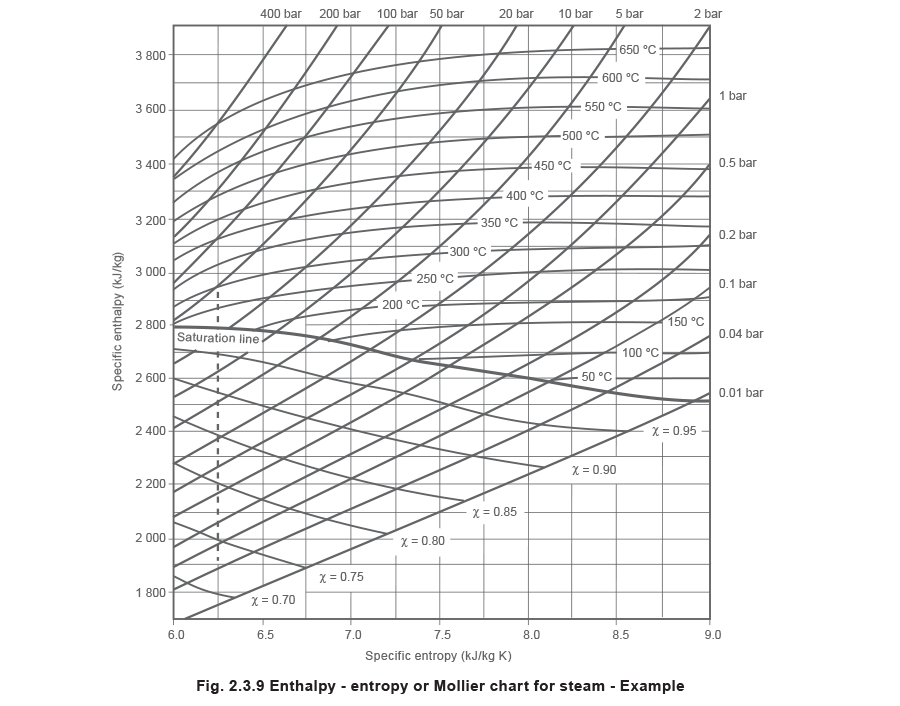
Since the entropy of dry saturated steam at 0.04 bar a (8.473 kJ/kg °C) is greater than the entropy of the superheated steam at 50 bar a/300 °C (6.212 kJ/kg °C), it follows that some of the dry out saturated steam must have condensed to maintain the abiding entropy.
Every bit the entropy remains constant, at the last condition:

These answers stand for closely with the results obtained using the Mollier nautical chart. The small difference in value between the 2 sets of results is to be expected, considering the inaccuracies involved in reading off a chart such as this.
Source: https://www.spiraxsarco.com/learn-about-steam/steam-engineering-principles-and-heat-transfer/superheated-steam
Posted by: thorntonhishad.blogspot.com

0 Response to "What Is The Change In The Specific Entropy Of Superheated R134a At 130"
Post a Comment